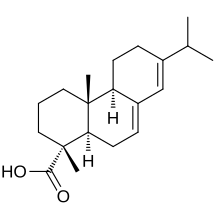
Abietic acid
| |
| |
| Names | |
|---|---|
| IUPAC name
Abieta-7,13-dien-18-oic acid
| |
| Systematic IUPAC name
(1R,4aR,4bR,10aR)-1,4a-Dimethyl-7-(propan-2-yl)-1,2,3,4,4a,4b,5,6,10,10a-decahydrophenanthrene-1-carboxylic acid | |
| Other names
Abietinic acid; Sylvic acid
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.007.436 |
| EC Number |
|
| KEGG | |
PubChem CID
|
|
| RTECS number |
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C20H30O2 | |
| Molar mass | 302.458 g·mol−1 |
| Appearance | Yellow resinous powder, crystals or chunks. Monoclinic plates (from EtOH/water). Colorless solid when pure. |
| Density | 1.06 g/mL |
| Melting point | 172–175 °C (342–347 °F; 445–448 K)[2] |
| Boiling point | 250 °C; 482 °F; 523 K |
| Insoluble[2] | |
| Solubility in other solvents | Very soluble in acetone, petroleum ether, Et2O, and ethanol |
| Hazards | |
| Occupational safety and health (OHS/OSH): | |
Main hazards
|
Irritant |
| GHS labelling: | |

| |
| Warning | |
| H317 | |
| P261, P272, P280, P302+P352, P321, P333+P313, P363, P501 | |
| NFPA 704 (fire diamond) | |
| Safety data sheet (SDS) | MSDS |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Abietic acid (also known as abietinic acid or sylvic acid) is a diterpenoid found in coniferous trees. It is supposed to exist as a defend the host plant from insect attack or various wounds. Chemically, it is a complicated molecule featuring two alkene groups and a carboxylic acid within a chiral tricyclic framework. As the major component of rosin, it is a commercially important. Historically speaking, it was a major component of naval stores. It is the most common of the resin acids. Another common resin acid is pimaric acid, which converts to abietic acid upon heating.
Characteristics
[edit]Abietic acid is found in rosin obtained from pine trees.[3] Pure abietic acid is a colorless solid, but commercial samples are usually a glassy or partly crystalline yellowish solid that melts at temperatures as low as 85 °C (185 °F).[4]
Abietic acid is soluble in alcohols, acetone, and ethers. Its ester or soap is called an abietate.[5]
Biosynthesis
[edit]
Abietic acid is derived from the diterpene abietane, which in turn is made from copalyl pyrophosphate, which is derived from the precursor to many diterpenoids, geranylgeranyl pyrophosphate. In air and in the presence of certain cytochrome P450 enzymes, abietane is oxidized to abietic acid. An entire family of so-called resin acid form similarly. Together with abietic acid, these resin acids are a major portion of rosin, the solid portion of the oleoresin of coniferous trees.
Preparation
[edit]Abietic acid is extracted from tree rosin. Laboratory procedures illustrate the nature of the extraction, which is the basic of a large industry.[6]
Uses
[edit]As a component of rosin and one of the principal resin acid]]s, abietic acid has many uses, e.g. in some paints, soaps, foods, soldering flux,
Safety
[edit]- As the chief component of rosin, abietic acid is approved by the US FDA as a miscellaneous food additive.[7]
- Abietic acid is considered a "nonhazardous natural substance" in tall oil ("liquid rosin").[5]
- In the U.S., abietic acid is listed in the inventory of the Toxic Substances Control Act.
- Abietic acid is the primary irritant in pine wood and resin. As a contact allergen[8] it is the cause of abietic acid dermatitis. (However, compounds resulting from its oxidation by air elicit stronger responses.) [9]
- 50% ethanol extracts from Resina pini of Pinus sp. (Pinaceae) showed inhibitory activity against testosterone 5α-reductase prepared from rat prostate. The fraction responsible for this activity was purified, and the active constituent was isolated and identified as abietic acid, which exhibited potent inhibitory activity against testosterone 5α-reductase in vitro.[10]
References
[edit]- ^ National Toxicology Program, Institute of Environmental Health Sciences, National Institutes of Health (NTP). 1992. National Toxicology Program Chemical Repository Database. Research Triangle Park, North Carolina
- ^ a b Merck Index, 12th Edition, 3. Abietic Acid
- ^ "Abietic Acid". Dr. Duke's Phytochemical and Ethnobotanical Databases. Archived from the original on 2015-09-23. Retrieved 13 January 2012.
- ^ Cite error: The named reference
EBwas invoked but never defined (see the help page). - ^ a b Lars-Hugo Norlin "Tall Oil" in Ullmann's Encyclopedia of Industrial Chemistry 2002, Wiley-VCH, Weinheim.doi:10.1002/14356007.a26_057
- ^ G. C. Harris and T. F. Sanderson (1963). "Abietic Acid". Organic Syntheses. 32: 1. doi:10.15227/orgsyn.032.0001.
- ^ Cite error: The named reference
:0was invoked but never defined (see the help page). - ^ El Sayed, F; Manzur, F; Bayle, P; Marguery, MS; Bazex, J (1995). "Contact urticaria from abietic acid". Contact Dermatitis. 32 (6): 361–2. doi:10.1111/j.1600-0536.1995.tb00628.x. PMID 7554886. S2CID 36139468.
- ^ Hausen, BM; Krohn, K; Budianto, E (1990). "Contact allergy due to colophony (VII). Sensitizing studies with oxidation products of abietic and related acids". Contact Dermatitis. 23 (5): 352–8. doi:10.1111/j.1600-0536.1990.tb05171.x. PMID 2096024. S2CID 34726630.
- ^ Seong-Soo Roh, Moon-Ki Park and Yong-ung Kim (2010). "Abietic Acid from Resina Pini of Pinus Species as a Testosterone 5α-Reductase Inhibitor". J. Health Sci. 56 (4): 451–455. doi:10.1248/jhs.56.451.

